The quantitative conversion of polyethylene terephthalate (PET) and Coca-Cola bottles to p-xylene over Co-based catalysts with tailored activities for deoxygenation and hydrogenation†
Abstract
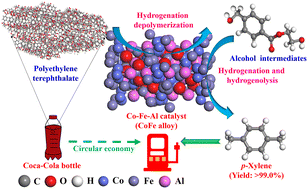
The selective depolymerization of PET plastics to p-xylene (xylene) is very challenging owing to the full deoxygenation of the terephthalic acid monomer and the retainment of the benzene ring through saturation. In this study, the modification of Co–Al catalysts by introducing a second metal (Fe, Cu, Ni, or Zn) was conducted to tailor the activity of the cobalt species for the conversion of PET to xylene. The results indicated that the CoFe alloy in the Co–Fe–Al catalyst weakened the adsorption/activation of H2, achieving xylene yields of >99.0% from PET. Co–Fe–Al suppressed the ring hydrogenation of the benzene ring, while Co–Al and Co–Ni–Al catalyzed the hydrogenation of the benzene ring and cleavage of the C–C bond in the intermediates. Benzene ring-containing intermediates showed a high affinity for Co–Ni–Al and Co–Al, facilitating their hydrogenation. Co–Fe–Al could preferably adsorb and activate the oxygen-containing functionalities of the intermediates for the deoxygenation reactions. The hydrogenolysis reaction of C–OH in intermediates, like 1,4-benzenedimethanol (Ea: 137.8 kJ mol−1), was the rate-determining step, while the hydrogenation of the carboxylic intermediates was much easier.


 Please wait while we load your content...
Please wait while we load your content...