A closed-loop process for high-value regeneration of spent LiFePO4 cathodes after selective aluminium precipitation
Abstract
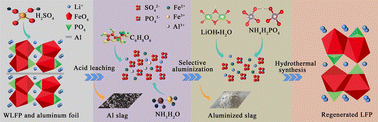
Regarding issues such as the removal of Al and resource utilization of phosphorus iron, a recycling process is proposed in this paper in which spent cathode materials and Al foil are leached by low-concentration acids, then Al is selectively precipitated, and finally the lithium iron phosphate material is synthesized by a one-step hydrothermal synthesis method after being prepared in a certain liquid ratio. Each influencing factor in the processes of acid leaching and precipitation of Al was investigated, and the corresponding mechanism and elemental direction were analyzed. The experimental results showed that the ideal leaching effect could be achieved using low concentrations of sulfuric acid, at leaching rates of 99.98%, 99.12%, 99.16%, and 22.44% for lithium, iron, phosphorus, and Al, respectively, under optimal conditions. Al was selectively precipitated in the form of Al phosphate, and the removal rate could reach more than 99.71%. This experimental scheme significantly reduces the necessary amounts of acid and alkali, produces less slag, is carried out using a compact process, and realizes the efficient recycling of lithium iron phosphate resources.
- This article is part of the themed collection: 2023 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...