CO2-derived non-isocyanate polyurethanes (NIPUs) and their potential applications
Abstract
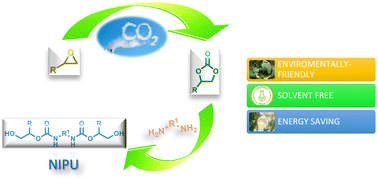
Using CO2 as feedstock to fabricate valuable products has become essential to green and sustainable chemistry and represents a rewarding challenge. Among the noticeable routes used to convert CO2 into synthetic polymers, this review highlights the reactions concerning the cycloaddition of epoxides with CO2 in cyclic carbonate as precursors for various forms of chemical synthesis, such as polycarbonates and polyurethanes (PUs). It is a fundamental challenge in polymer production to exploit biomass and CO2 as feedstock. PUs are one of the most versatile classes of polymeric materials that exhibit excellent properties. PUs are usually synthesized by a route involving the reaction of diols with diisocyanates derived from toxic phosgene gas. Non-isocyanate-derived polyurethane (NIPU) has been produced from cyclic carbonates and diamines without isocyanate. NIPU usually displays increased chemical resistance, lower permeability, improved water absorption, and thermal stability. In this review, we report the synthesis of several NIPUs for different applications in a more environmentally friendly manner, employing CO2 as a reagent and/or fully biobased reactants.
- This article is part of the themed collection: 2023 Green Chemistry Reviews


 Please wait while we load your content...
Please wait while we load your content...