Electrochemical N-acylation and N-α-ketoacylation of sulfoximines via the selective decarboxylation and dehydration of α-ketoacids†
Abstract
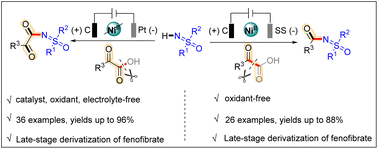
Sulfoximines are commonly found in pharmaceuticals, agrochemicals and other biologically active compounds. However, limited reports exist for their selective functionalization via green and efficient protocols. Herein, we describe an electrochemical N-acylation and N-α-ketoacylation of sulfoximines via the selective decarboxylation and dehydration of α-ketoacids. These two reactions use electricity as a “traceless” oxidant and α-ketoacid as a selective “acyl” or “α-ketoacyl” source. A broad range of acylated- and α-ketoacylated sulfoximines were isolated in good to excellent yields (up to 88% and 96% yields). Moreover, these protocols have also been applied to late-stage derivatizations of fenofibrate and can be safely conducted on a gram scale.


 Please wait while we load your content...
Please wait while we load your content...