Electrochemical cascade sequences for remote C7–H bond thiocyanation of quinoxalin-2(1H)-ones with ammonium thiocyanate†
Abstract
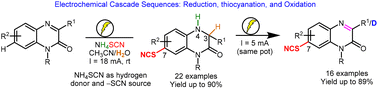
The syntheses of C3-substituted quinoxalin-2(1H)-one derivatives have been extensively studied for decades, while the methods for direct functionalization at the embedded aromatic ring have rarely been achieved. Herein, it is reported that such a remote C–H functionalization can be enabled by electrochemical cascade sequences. Thus, quinoxalin-2(1H)-ones can be converted into 7-thiocyanatoquinoxalin-2(1H)-ones in a CH3CN/H2O solvent mixture by using an undivided cell at a constant current with inexpensive graphite electrodes and commercially cheap ammonium thiocyanate as the thiocyanate source. These cascade sequences consist of sequential cathodic reduction at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond utilizing NH4SCN and H2O as the hydrogen atom donors, regioselective oxidative C7-thiocyanation, and anodic oxidation of the C–N single bond. The proposed mechanism has been supported by detailed cyclic voltammetry studies and control experiments. This oxidant-free strategy not only offers benefits in terms of sustainability and efficiency toward excellent functional group compatibility but also enables the synthesis of C3-deuterated derivatives.
N double bond utilizing NH4SCN and H2O as the hydrogen atom donors, regioselective oxidative C7-thiocyanation, and anodic oxidation of the C–N single bond. The proposed mechanism has been supported by detailed cyclic voltammetry studies and control experiments. This oxidant-free strategy not only offers benefits in terms of sustainability and efficiency toward excellent functional group compatibility but also enables the synthesis of C3-deuterated derivatives.


 Please wait while we load your content...
Please wait while we load your content...