Visible light photocatalytic phosphorylation of heteroatom nucleophiles triggered by phosphorus-centered radical cations†
Abstract
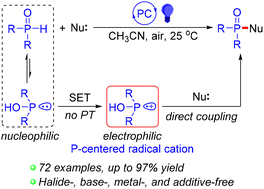
The development of P(O)–heteroatom bond-forming reactions is of vital importance. However, previous methods all have their own limitations and drawbacks. Herein, we disclose an efficient, green, and direct photocatalytic phosphorylation of heteroatom nucleophiles under the irradiation of 12 W blue LEDs at room temperature using acid red 51 as the photocatalyst and eco-friendly air as the sole oxidant. The newly developed reaction shows broad substrate scope under halide-, base-, metal-, and additive-free conditions, providing practical and scalable access to phosphinic fluorides, phosphinic amides, phosphoramidates, and phosphinates. Mechanistic studies on the phosphorylation reaction suggest that a phosphorus-centered radical cation generated from the P(O)–H reagent via a photocatalytic single electron transfer (SET) might be the main reactive species involved in this transformation.


 Please wait while we load your content...
Please wait while we load your content...