Rapid dissolution of high concentration poly(3-hydroxybutyrate) using neoteric biosolvents: experiment and molecular dynamics simulation†
Abstract
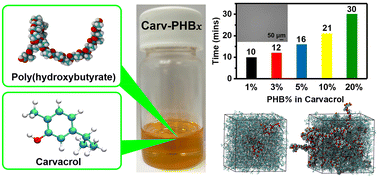
Nature, especially in hot environments, triggers the phase change of the abundant metabolites in plants from solid to liquid as a survival mechanism. Therefore, taking cues from nature, these metabolites could have potential as green solvents for the dissolution of biopolymers. Because of their green credentials, plant-derived aromatic monoterpenoids have gained considerable attention for the extraction of bioactive compounds to replace toxic halogenated solvents. In this work, we report for the first time the fast dissolution of high concentration poly(3-hydroxybutyrate) (PHB) using carvacrol (5-isopropyl-2-methylphenol) – a plant metabolite – as a solvent. Remarkably, 20 wt% PHB was completely dissolved within 30 min at 100 °C, whereas a 10 wt% PHB dissolved in 21 min, and even faster dissolution for 1 wt%, 3 wt% and 5 wt% PHB. The carvacrol/PHB solution exhibited stability characterized by rheology measurements and the atomistic molecular dynamics (MD) simulations elucidate the mechanism behind carvacrol's rapid dissolution of PHB. Most importantly, the carvacrol solvent can be fully recovered by adding anti-solvents. Furthermore, the resultant highly viscous carvacrol/PHB solution could find applications in coating formulations, adhesives, and consumer care additives.


 Please wait while we load your content...
Please wait while we load your content...