Selective C–C bond cleavage of oxidized lignin in an aqueous phase under mild conditions†
Abstract
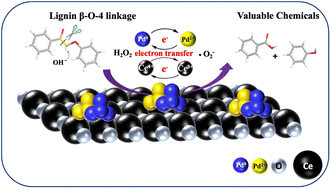
Selective cleavage of C–C bonds in the lignin structure is crucial for the depolymerization of lignin and producing value-added chemicals. In this study, we reported that Pd/CeO2 catalysts can selectively catalyze the Cα–Cβ bond cleavage of oxidized lignin model components and authentic lignin in an aqueous phase under mild conditions (50 °C, ambient pressure). A variety of β-O-4 and β-1 ketones with representative Cα–Cβ bonds were successfully cleaved yielding aromatic acids and phenols in 76.8–94.9% yields. Their unique catalytic properties are derived from the electron transfer between Pd and CeO2 support which facilitates active Pd2+ sites and reactive oxygen species formation. A mechanistic study elucidates that base-mediated C–H bond activation via enolization and in situ reactive oxygen species (˙O2−) formation are the key steps for the selective Cα–Cβ cleavage. Furthermore, the applicability of the system for the bond cleavage in an authentic lignin sample was demonstrated. Nearly 54.2 wt% of oxidized bamboo lignin was converted, generating aromatic monomer yields up to 35.9 wt%, with a high selectivity for aromatic aldehydes.


 Please wait while we load your content...
Please wait while we load your content...