Speeding up sustainable solution-phase peptide synthesis using T3P® as a green coupling reagent: methods and challenges†
Abstract
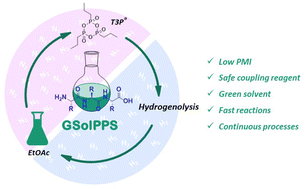
In peptide synthesis, the issues related to poor sustainability, long reaction times and high process mass intensity (PMI) are necessary to promote actions aimed at redefining procedural aspects projected towards more sustainable synthetic processes. Herein, we report a fast, widely applicable and green solution-phase peptide synthesis (GSolPPS) via a continuous protocol using propylphosphonic anhydride T3P® as the coupling reagent and N-benzyloxycarbonyl-protecting group (Z), which is easily removed by hydrogenation. Because N,N-dimethylformamide (DMF) replacement was a priority, the iterative process was performed in EtOAc, pushing further on overall sustainability. The efficiency of the synthetic protocol in terms of conversion, racemization and reaction times allowed extending the scope of the work to the synthesis of the standard peptide Leu-enkephalin as a proof of concept. Among the various explored procedures, the one-pot protocol (Acont plus), avoiding work-ups, intermediate purification and any dispersion effect, allowed the achievement of PMI = 30 for each deprotection/coupling sequence necessary to introduce a single amino acid in the iterative process, without considering the possibility of solvent and base recovery. This value is the lowest reported for an oligopeptide synthesis protocol to date.


 Please wait while we load your content...
Please wait while we load your content...