Metal-free cysteamine-functionalized graphene alleviates mutual interferences in heavy metal electrochemical detection†
Abstract
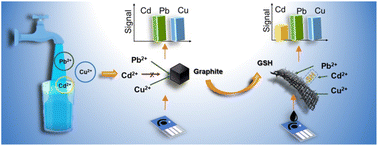
Heavy metal pollutants are of great concern to environmental monitoring due to their potent toxicity. Electrochemical detection, one of the main techniques, is hindered by the mutual interferences of various heavy metal ions in practical use. In particular, the sensitivity of carbon electrodes to Cd2+ ions (one of the most toxic heavy metals) is often overshadowed by some heavy metals (e.g. Pb2+ and Cu2+). To mitigate interference, metallic particles/films (e.g. Hg, Au, Bi, and Sn) typically need to be embedded in the carbon electrodes. However, these additional metallic materials may face issues of secondary pollution and unsustainability. In this study, a metal-free and sustainable nanomaterial, namely cysteamine covalently functionalized graphene (GSH), was found to lead to a 6-fold boost in the Cd2+ sensitivity of the screen-printed carbon electrode (SPCE), while the sensitivities to Pb2+ and Cu2+ were not influenced in simultaneous detection. The selective enhancement could be attributed to the grafted thiols on GSH sheets with good affinity to Cd2+ ions based on Pearson's hard and soft acid and base principle. More intriguingly, the GSH-modified SPCE (GSH-SPCE) featured high reusability with extended cycling times (23 times), surpassing the state-of-art SPCEs modified by non-covalently functionalized graphene derivatives. Last, the GSH-SPCE was validated in tap water.


 Please wait while we load your content...
Please wait while we load your content...