Vanillin-derived α,ω-diene monomer for thermosets preparation via thiol–ene click polymerization†
Abstract
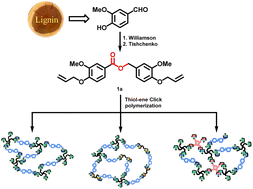
It is of great significance for sustainable development to produce degradable, thiol–ene networks obtained from bio-based monomers using environment-friendly processes. Hence, we have prepared tunable and degradable thiol–ene crosslinked networks from renewable vanillin and multifunctional thiols. Vanillin was converted into an α,ω-diene monomer (1a) with degradable ester group via Williamson and Tishchenko reactions. UV-thermally induced thiol–ene click polymerizations between 1a and thiols with various functionalities were performed to prepare thiol–ene networks. 1,2-Ethanedithiol and 2,4,6,8-tetramethyl-2,4,6,8-tetravinylcyclotetrasiloxane were also incorporated into the networks to further regulate their properties. The curing behaviours were studied by FT-IR and DSC to determine the optimal curing conditions. The thermomechanical, thermal and mechanical characteristics of the networks were investigated by DMA, DSC, TGA, and tensile strength tests to understand the influence of different crosslinkers on the material's properties. By varying the amount of co-monomer, the networks exhibited glass transition temperature, tensile strength, and elongations at break in the ranges of 14.5–43.3 °C, 2.6–24.8 MPa, and 29.1–510.2%, respectively. All the thiol–ene networks had good transparency with transmittance between 85 and 90% at 600 nm. Moreover, the networks could be degraded in mild alkaline aqueous solution owing to their inherent ester groups.


 Please wait while we load your content...
Please wait while we load your content...