Retracted Article: Stereoisomers of octahydrocurcumin, the hydrogenated metabolites of curcumin, display stereoselective activity on the CYP2E1 enzyme in L-02 cells
Abstract
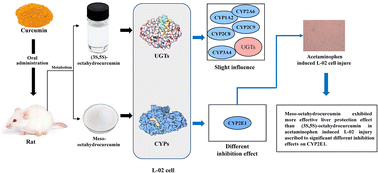
As the final hydrogenated metabolite of curcumin, octahydrocurcumin (OHC) exhibits increased powerful bioactivities. The chiral and symmetric chemical structure indicated that there were two OHC stereoisomers, (3R,5S)-octahydrocurcumin (Meso-OHC) and (3S,5S)-octahydrocurcumin ((3S,5S)-OHC), which may induce different effects on metabolic enzymes and bioactivities. Thus, we detected OHC stereoisomers from rat metabolites (blood, liver, urine and feces) after oral administration of curcumin. In addition, OHC stereoisomers were prepared and then their different influences on cytochrome P450 enzymes (CYPs) and UDP-glucuronyltransferases (UGTs) in L-02 cells were tested to explore the potential interaction and different bioactivities. Our results proved that curcumin could be metabolised into OHC stereoisomers first. In addition, Meso-OHC and (3S,5S)-OHC exhibited slight induction or inhibition effects on CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP3A4 and UGTs. Furthermore, Meso-OHC exhibited more intensive inhibition toward CYP2E1 expression than (3S,5S)-OHC, ascribed to the different mode of binding to the enzyme protein (P < 0.05), which finally induced more effective liver protection effects in acetaminophen-induced L-02 cell injury.


 Please wait while we load your content...
Please wait while we load your content...