Impact of sodium pyruvate on the electrochemical reduction of NAD+ biomimetics†
Abstract
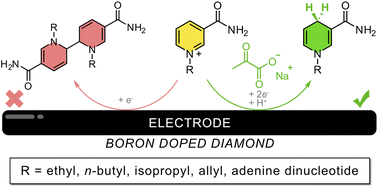
Biomimetics of nicotinamide adenine dinucleotide (mNADH) are promising cost-effective alternatives to their natural counterpart for biosynthetic applications; however, attempts to recycle mNADH often rely on coenzymes or precious metal catalysts. Direct electrolysis is an attractive approach for recycling mNADH, but electrochemical reduction of the oxidized mimetic (mNAD+) primarily results in the formation of an enzymatically inactive dimer. Herein, we find that aqueous electrochemical reduction of an NAD+ mimetic, 1-n-butyl-3-carbamoylpyridinium bromide (1+), to its enzymatically active form, 1,4-dihydro-1-n-butyl nicotinamide (1H), is favored in the presence of sodium pyruvate as a supporting electrolyte. Maximum formation of 1H is achieved in the presence of a large excess of pyruvate in combination with a large excess of a co-supporting electrolyte. Formation of 1H is found to be favored at pH 7, with an optimized product ratio of ∼50/50 dimer/1H observed by cyclic voltammetry. Furthermore, sodium pyruvate is shown to promote electroreductive generation of the 1,4-dihydro form of several additional mNADH as well as NADH itself. This method provides a general strategy for regenerating 1,4-dihydro-nicotinamide mimetics of NADH from their oxidized forms.
- This article is part of the themed collection: Electrosynthesis


 Please wait while we load your content...
Please wait while we load your content...