Detailed-balance limits for sunlight-to-protonic energy conversion from aqueous photoacids and photobases based on reversible mass-action kinetics†
Abstract
Detailed-balance limits to energy conversion efficiency critically inform design rules for photochemical power conversion devices. Herein we simulate efficiencies for sunlight-to-protonic power conversion for liquid water, which serves as the protonic semiconductor and is sensitized to visible-light absorption by reversible photoacids or photobases. Our model includes proton-transfer processes based on the Förster cycle with rate constants that follow the empirical Brønsted relation, where bimolecular reactions are encounter controlled. Based on physically relevant model parameters, simulations of steady-state concentrations of H+(aq) and OH−(aq) indicate that for defect-free water the maximum possible protonic quasi-chemical potentials result in a photovoltage of ∼330 mV and a power conversion efficiency of ∼10%. Conditions of maximum power conversion occur when photoacid (photobase) dyes exhibit acidities (basicities) of pKa (pKb) ≥ 14 and 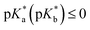 , an outcome that is nearly independent of equilibrium pH. These conditions are optimal because under standard-state conditions they result in rates for protonation of water to form H+(aq) and deprotonation of water to form OH−(aq) that are faster than other proton-transfer processes, due to their isoergic/exoergic nature. Simulations also indicate that longer excited-state lifetimes result in an increase in the range of pK* values that lead to significant protonic quasi-chemical potentials. This occurs because longer excited-state lifetimes up to ∼1 μs afford more time to perform excited-state proton transfer. Simulation outcomes are affected little by the inclusion of empirical nonzero activation free energies for isoergic/exoergic proton-transfer reactions or rate constants for equilibrium radiative generation and recombination of excited-state species under thermal detailed balance. Only when local electric fields are assumed present to increase rate constants for proton-transfer reactions do protonic quasi-chemical potentials suffer. Simulations also indicate that the total concentration of photoacid or photobase dyes exhibits two competing effects on protonic quasi-chemical potentials. Decreasing dye concentration results in less sunlight absorption and therefore smaller changes in steady-state concentrations of H+(aq) and OH−(aq), while also increasing the range of pK values that results in significant changes in quasi-chemical potentials. Overall, these results define parameters for effective sunlight-to-protonic power conversion and will help guide researchers in the design and development of photoacid and photobase dyes for light-driven proton pumps.
, an outcome that is nearly independent of equilibrium pH. These conditions are optimal because under standard-state conditions they result in rates for protonation of water to form H+(aq) and deprotonation of water to form OH−(aq) that are faster than other proton-transfer processes, due to their isoergic/exoergic nature. Simulations also indicate that longer excited-state lifetimes result in an increase in the range of pK* values that lead to significant protonic quasi-chemical potentials. This occurs because longer excited-state lifetimes up to ∼1 μs afford more time to perform excited-state proton transfer. Simulation outcomes are affected little by the inclusion of empirical nonzero activation free energies for isoergic/exoergic proton-transfer reactions or rate constants for equilibrium radiative generation and recombination of excited-state species under thermal detailed balance. Only when local electric fields are assumed present to increase rate constants for proton-transfer reactions do protonic quasi-chemical potentials suffer. Simulations also indicate that the total concentration of photoacid or photobase dyes exhibits two competing effects on protonic quasi-chemical potentials. Decreasing dye concentration results in less sunlight absorption and therefore smaller changes in steady-state concentrations of H+(aq) and OH−(aq), while also increasing the range of pK values that results in significant changes in quasi-chemical potentials. Overall, these results define parameters for effective sunlight-to-protonic power conversion and will help guide researchers in the design and development of photoacid and photobase dyes for light-driven proton pumps.



 Please wait while we load your content...
Please wait while we load your content...