Tailoring acidic microenvironments for carbon-efficient CO2 electrolysis over a Ni–N–C catalyst in a membrane electrode assembly electrolyzer†
Abstract
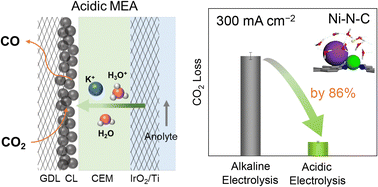
CO2 electrolysis converting CO2 into valuable fuels and chemicals powered by renewable electricity shows great promise for practical applications. However, it suffers from low energy efficiency and carbon efficiency due to severe carbon loss in alkaline and neutral electrolytes. Here we present a carbon-efficient CO2 electrolysis strategy using a zero-gap acidic membrane electrode assembly electrolyzer. The microenvironments of a Ni–N–C catalyst (such as local concentrations of H+/K+ and CO2) are turned by optimizing the anolyte composition (pH, K+) and input CO2 pressure. Under optimal conditions, acidic CO2 electrolysis over the Ni–N–C catalyst achieves a CO faradaic efficiency of 95% at a total current density of 500 mA cm−2, corresponding to a CO production rate as high as 13 mL min−1. An energy efficiency of 45% for CO production is obtained at pH 0.5 and the CO2 loss is reduced by 86% at 300 mA cm−2, compared to alkaline CO2 electrolysis. Density functional theory calculations reveal that the co-existence of H+ and K+ plays a crucial role in stabilizing the initial *CO2 intermediate, resulting in enhanced CO formation.


 Please wait while we load your content...
Please wait while we load your content...