Energy-efficient electrochemical ammonia production from dilute nitrate solution†
Abstract
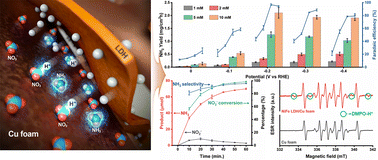
Highly efficient electrochemical nitrate reduction could become a key process for sustainable ammonia production overcoming many limitations of the Haber–Bosch process. Current state-of-the-art electrocatalysts have severe drawbacks regarding yield, selectivity and energy efficiency when dealing with dilute nitrate solutions. Herein, we report a layered double hydroxide (LDH)/Cu foam hybrid electrocatalyst that offers a potential solution to this challenge. The [Ni0.75Fe0.25(OH)2](CO3)0.125 (Ni3Fe–CO3 LDH) exhibits an appropriate kinetic energy barrier for the Volmer step generating hydrogen radicals as well as suppressing H–H bond formation by inhibition of the Heyrovsky step. The electrochemically generated hydrogen radicals transfer to a Cu surface enabling NO3− reduction to NH3. The Ni3Fe–CO3 LDH/Cu foam hybrid electrode exhibits an 8.5-fold higher NH3 yield compared to a pristine Cu surface, while exhibiting an NH3 selectivity of 95.8% at 98.5% NO3− conversion. The best half-cell energy efficiency (36.6%) was recorded while achieving 96.8% faradaic efficiency at −0.2 V in 5 mM NO3−(aq).
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...