Optimization of oxygen evolution activity by tuning e *g band broadening in nickel oxyhydroxide†
Abstract
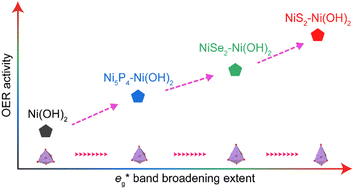
Nickel oxyhydroxides (NiOOH) derived from the reconstruction of Ni-based pre-catalysts are active species for the oxygen evolution reaction (OER). Although chemically similar, these NiOOH exhibit differentiated OER activities that show strong correlations to their synthetic origins. To unearth the mechanism behind this, three NiOOH were prepared by subjecting NiS2, NiSe2 and Ni5P4 to chronopotentiometry treatment. We found that a stronger strain leads to a greater NiO6 octahedron distortion in NiOOH, which results in the broadening of the 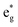 band (3d electron states with eg symmetry) to a great extent. The increase in
band (3d electron states with eg symmetry) to a great extent. The increase in 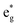 band broadening can facilitate electron transfer from the electrocatalysts to the external circuit, which ultimately enhances the catalytic performance. We demonstrated the universality of this concept by extending it to a NiFe oxyhydroxide system. This study shows the first evidence of the relationship between structure and catalytic performance by revealing the role of
band broadening can facilitate electron transfer from the electrocatalysts to the external circuit, which ultimately enhances the catalytic performance. We demonstrated the universality of this concept by extending it to a NiFe oxyhydroxide system. This study shows the first evidence of the relationship between structure and catalytic performance by revealing the role of 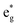 band broadening, paving the way toward designing efficient OER electrocatalysts.
band broadening, paving the way toward designing efficient OER electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...