Ring-opening yields and auto-oxidation rates of the resulting peroxy radicals from OH-oxidation of α-pinene and β-pinene†
Abstract
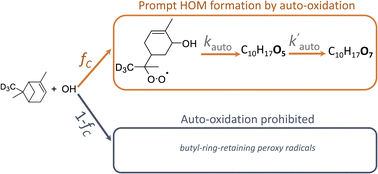
Atmospheric oxidation of monoterpenes (C10H16) contributes to ambient particle number and mass concentrations due, in part, to the resulting peroxy radicals undergoing auto-oxidation to low-volatility highly oxygenated molecules (HOMs). Only one of the structural isomers of the first-generation hydroxy peroxy radical (C10H17O3), that formed with an opening of the cyclobutyl ring, is thought to be responsible for the majority of HOMs derived from OH-initiated oxidation of α and β-pinene. We constrain the yield and auto-oxidation rate of this isomer by using a unique combination of isotopically labeled precursors, such as D3-α-pinene, and direct measurements of peroxy radicals and closed-shell products after a reaction time of only ∼0.7 s. Using competitive rate studies and quantum chemical calculations, we find that the yields of these ring-opened peroxy radicals are 0.47 (0.2, 0.6) for α-pinene and 0.66 (0.4, 0.8) for β-pinene, and the rates of auto-oxidation are 2.9 (1.1, 4.7) s−1 and 0.8 (0.4, 1.6) s−1, respectively, where values in the parentheses represent upper and lower bounds based on experimental and mechanistic uncertainties. These rates and yields are in general agreement with HOM yields determined from independent methods and in a range where OH driven monoterpene HOM formation will be atmospherically important for a wide set of conditions.


 Please wait while we load your content...
Please wait while we load your content...