Efficient and well-controlled ring opening polymerization of biobased ethylene brassylate by α-diimine FeCl3 catalysts via a coordination–insertion mechanism†
Abstract
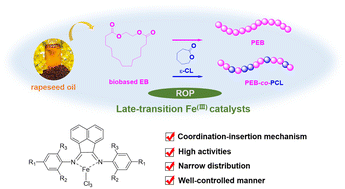
A highly efficient late-transition metal based catalytic system of α-diimine FeCl3 for well-controlled ring opening polymerization of a cheap and biobased macrolactone, ethylene brassylate (EB), is described herein. Proceeding via a coordination–insertion mechanism, such a catalytic system is capable of demonstrating unprecedented higher activities than previously reported organocatalysts or main-group metal based catalysts. Moreover, benefiting from the bulky nature of the α-diimine ligands, transesterification side reactions can be greatly suppressed, allowing the polymerization to proceed in a well-controlled living manner, as revealed from detailed kinetic studies. Additionally, such a catalytic system was also workable for ring opening copolymerization of EB and ε-caprolactone (ε-CL), giving the desired random copolymers with various compositions.


 Please wait while we load your content...
Please wait while we load your content...