Direct and remote control of electronic structures and redox potentials in μ-oxo diferric complexes†
Abstract
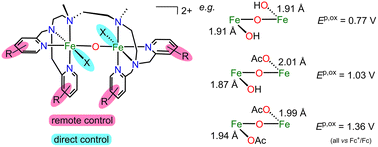
Non-heme diiron enzymes activate O2 for the oxidation of substrates in the form of peroxo FeIII2 or high-valent FeIV2 intermediates. We have developed a dinucleating bis(tetradentate) ligand system that stabilizes peroxo and hydroperoxo FeIII2 complexes with terminal 6-methylpyridine donors, while the peroxo FeIII2 intermediate is reactive with terminal pyridine donors presumably via conversion to a fluent high-valent FeIV2 intermediate. We present here a derivative with electron-donating methoxy substituents at the pyridine donors and its diferric complexes with an {FeIIIX(μ-O)FeIIIX} (X− = Cl−, OAc−, and OH−) or an {FeIII(μ-O)(μ-OAc)FeIII} core. The complex-induced oxidation of EtOH with H2O2 provides μ-OAc−, and in acetone, the complex with mixed OH−/OAc− exogenous donors is obtained. Both reactivities indicate a reactive fluent peroxo FeIII2 intermediate. The coupling constant J and the LMCT transitions are insensitive to the nature of the directly bound ligands X− and reflect mainly the electronic structure of the central {FeIII(μ-O)FeIII} core, while Mössbauer spectroscopy and d–d transitions probe the local FeIII sites. The remote methoxy substituents decrease the potential for the oxidation to FeIV by ∼100 mV, while directly bound OH− in {FeIII(OH)(μ-O)FeIII(OH)} with a short 1.91 Å FeIII–OOH bond decreases the potential by 590 mV compared to {FeIII(OAc)(μ-O)FeIII(OAc)} with a 2.01 Å FeIII–OOAc bond. Interestingly, this FeIII–OH bond is even shorter (1.87 Å) in the mixed OH−/OAc− complex but the potential is the mean value of the potentials of the OH−/OH− and OAc−/OAc− complexes, thus reflecting the electron density of the central {FeIII(μ-O)FeIII} core and not of the local FeIII–OH unit.


 Please wait while we load your content...
Please wait while we load your content...