Robust and efficient transfer hydrogenation of carbonyl compounds catalyzed by NN-Mn(i) complexes†
Abstract
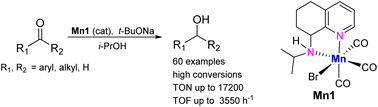
A series of manganese(I) carbonyl complexes bearing structurally related NN- and NNN-chelating ligands have been synthesized and assessed as catalysts for transfer hydrogenation (TH). Notably, the NN-systems based on N-R functionalized 5,6,7,8-tetrahydroquinoline-8-amines, proved the most effective in the manganese-promoted conversion of acetophenone to 1-phenylethanol. In particular, the N-isopropyl derivative, Mn1, when conducted in combination with t-BuONa, was the standout performer mediating not only the reduction of acetophenone but also a range of carbonyl substrates including (hetero)aromatic-, aliphatic- and cycloalkyl-containing ketones and aldehydes with especially high values of TON (up to 17 200; TOF of 3550 h−1). These findings, obtained through a systematic variation of the N-R group of the NN ligand, are consistent with an outer-sphere mechanism for the hydrogen transfer. As a more general point, this Mn-based catalytic TH protocol offers an attractive and sustainable alternative for producing alcoholic products from carbonyl substrates.


 Please wait while we load your content...
Please wait while we load your content...