UV induced hydrophosphination of dimethyl 2-vinylcyclopropane-1,1-dicarboxylate towards phosphine chalcogenides†
Abstract
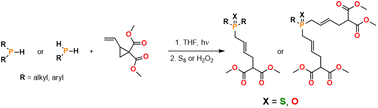
Dimethyl 2-vinylcyclopropane-1,1-dicarboxylate underwent a hydrophosphination reaction with either a primary or secondary phosphine under photolytic conditions. Notably, a free radical initiator was not required. The resulting tertiary phosphines were derivatized using S8 to afford moisture and air stable yellow or colorless oils in a 27%–73% isolated yield. A series of control reactions were performed, and we propose that this UV induced hydrophosphination reaction proceeds through a radical mechanism.


 Please wait while we load your content...
Please wait while we load your content...