Phase evolution, speciation and solubility limit of aluminium doping in zinc oxide catalyst supports synthesized via co-precipitated hydrozincite precursors†
Abstract
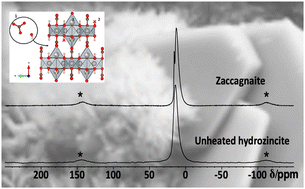
The preparation of Al-doped ZnO via thermal decomposition of crystalline precursors, with a particular emphasis on kinetic effects on the solubility limits, was studied. The promoting effect of Al3+ on the catalyst system is discussed for methanol synthesis where ZnO:Al is employed as a support material for copper nanoparticles. The synthesis of the Al-doped zinc oxides in this study was inspired by the industrial synthesis of the methanol synthesis catalyst via a co-precipitated crystalline precursor, here: hydrozincite Zn5(OH)6(CO3)2. To determine the aluminium speciation and the solubility limit of the aluminium cation on zinc positions, a series of zinc oxides with varying aluminium contents was synthesized by calcination of the precursors. Short precipitate ageing time, low ageing temperature and aluminium contents below 3 mol% metal were advantageous to suppress crystalline side-phases in the precursor, which caused an aluminium segregation and non-uniform aluminium distribution in the solid. Even if zinc oxide was the only crystalline phase, TEM revealed such segregation in samples calcined at 320 °C. Only at very low aluminium contents, the dopant was found preferably on the zinc sites of the zinc oxide structure based on the 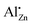 signal dominating the 27Al NMR spectra. The solubility limit regarding this species was determined to be approximately xAl = 0.013 or 1.3% of all metal cations. Annealing experiments showed that aluminium was kinetically trapped on the
signal dominating the 27Al NMR spectra. The solubility limit regarding this species was determined to be approximately xAl = 0.013 or 1.3% of all metal cations. Annealing experiments showed that aluminium was kinetically trapped on the 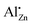 site and segregated into ZnAl2O4 upon further heating. This shows that lower calcination temperatures such as applied in catalyst synthesis conserve a higher aluminium doping concentration on that specific site than is expected thermodynamically.
site and segregated into ZnAl2O4 upon further heating. This shows that lower calcination temperatures such as applied in catalyst synthesis conserve a higher aluminium doping concentration on that specific site than is expected thermodynamically.


 Please wait while we load your content...
Please wait while we load your content...