Synthesis of NiFe layered double hydroxides with varied layer charge densities: the templating effect of dioctyl sulfosuccinate
Abstract
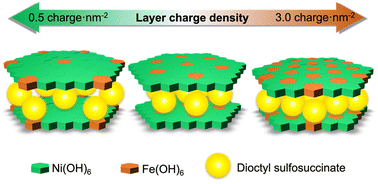
NiFe layered double hydroxides (LDHs) intercalated with dioctyl sulfosuccinate with varied Fe3+/(Ni2+ + Fe3+) ratios (0.04–0.25) were prepared by constant-pH co-precipitation from an aqueous solution of Ni and Fe perchlorates at room temperature. The interlayer dioctyl sulfosuccinate was exchanged with carbonate by the reaction of the product with an aqueous solution of sodium carbonate. The basal spacing of the NiFe-LDHs containing carbonate varied (0.80–0.90 nm) depending on the Fe3+/(Ni2+ + Fe3+) ratio; larger basal spacing was attained from the LDH with smaller Fe3+/(Ni2+ + Fe3+) due to the weaker attractive force between the LDH layer and the interlayer anion, proving that the Fe3+/(Ni2+ + Fe3+) ratios were associated with the layer charge density of NiFe-LDHs.


 Please wait while we load your content...
Please wait while we load your content...