Synthesis and structural, magnetic and spectroscopic characterization of iron(iii) complexes with in situ formed ligands from methyl-2-pyridyl ketone transformations†‡
Abstract
Reactions of methyl-2-pyridyl ketone, pyCOMe, with FeCl3·6H2O in various solvents gave complexes [Fe4Cl6(OMe)2(L1)2]·0.7MeCN·0.4MeOH (1·0.7MeCN·0.4MeOH) and [Fe3Cl4(bicine)(L2)]·Me2CO·0.2H2O (2·Me2CO·0.2H2O). The ligands (L1)2− = pyCO(Me)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COpy (in 1) and (L2)2− = pyCO(Me)CH2CO(OMe)py (in 2) are formed in situ, through an aldol reaction-type mechanism between the carbanion pyC(O)CH2− (formed by the nucleophilic attack of the MeO− in pyCOMe) and pyCOMe which results in the formation of a new C–C bond. The intermediate compound undergoes attack in the –CH2– or –C
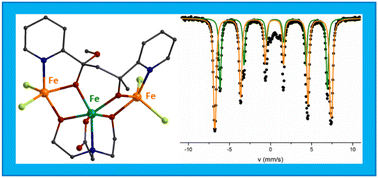
COpy (in 1) and (L2)2− = pyCO(Me)CH2CO(OMe)py (in 2) are formed in situ, through an aldol reaction-type mechanism between the carbanion pyC(O)CH2− (formed by the nucleophilic attack of the MeO− in pyCOMe) and pyCOMe which results in the formation of a new C–C bond. The intermediate compound undergoes attack in the –CH2– or –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– group by a MeO− group, and the new ligands (L1)2− and (L2)2−, respectively, are formed. The molecular structure of 1 consists of three corner-sharing [Fe2O2] rhombic units in cis-arrangement. The two terminal FeIII ions display distorted square pyramidal geometry and the two central FeIII ions are distorted octahedral. The molecular structure of 2 consists of two corner-sharing [Fe2O2] rhombic units, with the two terminal FeIII ions in distorted square pyramidal geometry and the central FeIII in distorted octahedral. The differentiation in the coordination environment of the FeIII ions in 1–2 is reflected in the values of the Mössbauer hyperfine parameters. In agreement with theoretical calculations, the square pyramidal sites exhibit a smaller isomer shift value in comparison to the octahedral sites. Magnetic studies indicate antiferromagnetic interactions leading to an S = 0 ground state in 1 and to an S = 5/2 ground state in 2, consistent with Electron Paramagnetic Resonance spectroscopy. Mössbauer spectra of 2 indicate the onset of relaxation effects below 80 K. At 1.5 K the spectrum of 2 consists of magnetic sextets. The determined hyperfine magnetic fields are consistent with the exchange coupling scheme imposed by the crystal structure of 2. Theoretical calculations shed light on the differences in the electronic structure between the square pyramidal and the octahedral sites.
O– group by a MeO− group, and the new ligands (L1)2− and (L2)2−, respectively, are formed. The molecular structure of 1 consists of three corner-sharing [Fe2O2] rhombic units in cis-arrangement. The two terminal FeIII ions display distorted square pyramidal geometry and the two central FeIII ions are distorted octahedral. The molecular structure of 2 consists of two corner-sharing [Fe2O2] rhombic units, with the two terminal FeIII ions in distorted square pyramidal geometry and the central FeIII in distorted octahedral. The differentiation in the coordination environment of the FeIII ions in 1–2 is reflected in the values of the Mössbauer hyperfine parameters. In agreement with theoretical calculations, the square pyramidal sites exhibit a smaller isomer shift value in comparison to the octahedral sites. Magnetic studies indicate antiferromagnetic interactions leading to an S = 0 ground state in 1 and to an S = 5/2 ground state in 2, consistent with Electron Paramagnetic Resonance spectroscopy. Mössbauer spectra of 2 indicate the onset of relaxation effects below 80 K. At 1.5 K the spectrum of 2 consists of magnetic sextets. The determined hyperfine magnetic fields are consistent with the exchange coupling scheme imposed by the crystal structure of 2. Theoretical calculations shed light on the differences in the electronic structure between the square pyramidal and the octahedral sites.
- This article is part of the themed collection: Spotlight Collection focused on Inorganic Chemistry in Greece


 Please wait while we load your content...
Please wait while we load your content...