Periodic trends in trivalent actinide halides, phosphates, and arsenates†
Abstract
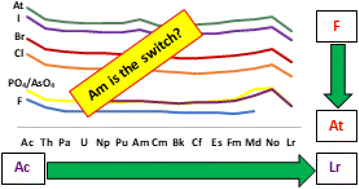
Due to the limited abundance of the actinide elements, computational methods, for now, remain an exclusive avenue to investigate the periodic trends across the actinide series. As every actinide element can exhibit a +3-oxidation state, we have explored model systems of gas-phase actinide trihalides, phosphates, and arsenates across the series to capture the periodic trends. By doing so, we were able to capture the periodic trends down the halogen series as well, and for the first time we are reporting a study on actinide astatides. Using scalar and spin–orbit relativistic Density Functional Theory (DFT) calculations, we have explored the variations in bond lengths, bond angles, and the charges on actinides (An). Despite the use of different sets of ligands, the trends remain similar. The properties of trivalent Pa, U, Np, and Pu are nearly identical; similar ionic radii could be the reason. The actinide elements show a tendency to exhibit a pre-Pu and a post-Cm behaviour, with Am acting as a switch. This could be due to the change in the behaviour from d–f-type to f-filling/d-type at around Pu–Cm in the actinides as already proposed in the previous literature. Bond lengths in the AnX3 increase down the halide series, and the atomic charges decrease on the actinide elements.


 Please wait while we load your content...
Please wait while we load your content...