Comparative oxidative ability of mononuclear and dinuclear high-valent iron–oxo species towards the activation of methane: does the axial/bridge atom modulate the reactivity?†
Abstract
Over the years, mononuclear FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species have been extensively studied, but the presence of dinuclear FeIV
O species have been extensively studied, but the presence of dinuclear FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species in soluble methane monooxygenase (sMMO) has inspired the development of biomimic models that could activate inert substrates such as methane. There are some successful attempts; particularly the [(Por)(m-CBA) FeIV(μ-N)FeIV(O)(Por˙+)]− species has been reported to activate methane and yield decent catalytic turnover numbers and therefore regarded as the closest to the sMMO enzyme functional model, as no mononuclear FeIV
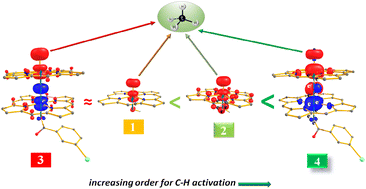
O species in soluble methane monooxygenase (sMMO) has inspired the development of biomimic models that could activate inert substrates such as methane. There are some successful attempts; particularly the [(Por)(m-CBA) FeIV(μ-N)FeIV(O)(Por˙+)]− species has been reported to activate methane and yield decent catalytic turnover numbers and therefore regarded as the closest to the sMMO enzyme functional model, as no mononuclear FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O analogues could achieve this feat. In this work, we have studied a series of mono and dinuclear models using DFT and ab initio DLPNO–CCSD(T) calculations to probe the importance of nuclearity in enhancing the reactivity. We have probed the catalytic activities of four complexes: [(HO)FeIV(O)(Por)]− (1), [(HO)FeIV(O)(Por˙+)] (2), μ-oxo dinuclear iron species [(Por)(m-CBA)FeIV(μ-O)FeIV(O) (Por˙+)]− (3) and N-bridged dinuclear iron species [(Por)(m-CBA)FeIV(μ-N)FeIV(O)(Por˙+)]− (4) towards the activation of methane. Additionally, calculations were performed on the mononuclear models [(X)FeIV(O)(Por˙+)]n {X = N 4a (n = −2), NH 4b (n = −1) and NH24c (n = 0)} to understand the role of nuclearity in the reactivity. DFT calculations performed on species 1–4 suggest an interesting variation among them, with species 1–3 possessing an intermediate spin (S = 1) as a ground state and species 4 possessing a high-spin (S = 2) as a ground state. Furthermore, the two FeIV centres in species 3 and 4 are antiferromagnetically coupled, yielding a singlet state with a distinct difference in their electronic structure. On the other hand, species 2 exhibits a ferromagnetic coupling between the FeIV and the Por˙+ moiety. Our calculations suggest that the higher barriers for the C–H bond activation of methane and the rebound step for species 1 and 3 are very high in energy, rendering them unreactive towards methane, while species 2 and 4 have lower barriers, suggesting their reactivity towards methane. Studies on the system reveal that model 4a has multiple Fe
O analogues could achieve this feat. In this work, we have studied a series of mono and dinuclear models using DFT and ab initio DLPNO–CCSD(T) calculations to probe the importance of nuclearity in enhancing the reactivity. We have probed the catalytic activities of four complexes: [(HO)FeIV(O)(Por)]− (1), [(HO)FeIV(O)(Por˙+)] (2), μ-oxo dinuclear iron species [(Por)(m-CBA)FeIV(μ-O)FeIV(O) (Por˙+)]− (3) and N-bridged dinuclear iron species [(Por)(m-CBA)FeIV(μ-N)FeIV(O)(Por˙+)]− (4) towards the activation of methane. Additionally, calculations were performed on the mononuclear models [(X)FeIV(O)(Por˙+)]n {X = N 4a (n = −2), NH 4b (n = −1) and NH24c (n = 0)} to understand the role of nuclearity in the reactivity. DFT calculations performed on species 1–4 suggest an interesting variation among them, with species 1–3 possessing an intermediate spin (S = 1) as a ground state and species 4 possessing a high-spin (S = 2) as a ground state. Furthermore, the two FeIV centres in species 3 and 4 are antiferromagnetically coupled, yielding a singlet state with a distinct difference in their electronic structure. On the other hand, species 2 exhibits a ferromagnetic coupling between the FeIV and the Por˙+ moiety. Our calculations suggest that the higher barriers for the C–H bond activation of methane and the rebound step for species 1 and 3 are very high in energy, rendering them unreactive towards methane, while species 2 and 4 have lower barriers, suggesting their reactivity towards methane. Studies on the system reveal that model 4a has multiple Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds facilitating greater reactivity, whereas the other two models have longer Fe–N bonds and less radical character with steeper barriers. Strong electronic cooperativity is found to be facilitated by the bridging nitride atom, and this cooperativity is suppressed by substituents such as oxygen, rendering them inactive. Thus, our study unravels that apart from enhancing the nuclearity, bridging atoms that facilitate strong cooperation between the metals are required to activate very inert substrates such as methane, and our results are broadly in agreement with earlier experimental findings.
N bonds facilitating greater reactivity, whereas the other two models have longer Fe–N bonds and less radical character with steeper barriers. Strong electronic cooperativity is found to be facilitated by the bridging nitride atom, and this cooperativity is suppressed by substituents such as oxygen, rendering them inactive. Thus, our study unravels that apart from enhancing the nuclearity, bridging atoms that facilitate strong cooperation between the metals are required to activate very inert substrates such as methane, and our results are broadly in agreement with earlier experimental findings.


 Please wait while we load your content...
Please wait while we load your content...