Hydrogen complexes on single atom alloys: classical chemisorption versus coordination chemistry†
Abstract
Single Atom Alloys (SAAs) represent one of the most promising classes of heterogeneous catalysts. They are based on isolated transition metal (TM) atoms stabilized in a host metal matrix. Among others, SAAs are widely studied for processes involving hydrogen, such as water splitting or hydrogenation reactions. On a metal surface the H2 molecule forms either weakly physisorbed species (H2phys) or dissociates with formation of chemisorbed H atoms (H*). In electrochemical processes, the adsorption free energy of the H* intermediate is normally used as a descriptor of the catalyst reactivity. Recently, it has been shown that on Single Atom Catalysts (SAC) embedded in carbon-based, sulfide, or oxide supports other species can form, where two H atoms are stably bound to the SAC, forming dihydrogen 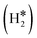 or dihydride (H*H*) surface species reminiscent of the classical Kubas's transition metal complexes in coordination chemistry. In this work we show, based on density functional theory calculations, that dihydrogen and dihydride complexes can also form on SAAs and, depending on the nature of the host metal, can be even more stable than two separated chemisorbed H* species (2H*). These new stable intermediates have not been considered so far in the analysis of the mechanism and kinetics of the reaction. The work shows that the possible formation of dihydrogen and dihydride complexes is not limited to SACs but is also valid for SAAs and needs to be considered in the study of hydrogen-based reactions on these systems.
or dihydride (H*H*) surface species reminiscent of the classical Kubas's transition metal complexes in coordination chemistry. In this work we show, based on density functional theory calculations, that dihydrogen and dihydride complexes can also form on SAAs and, depending on the nature of the host metal, can be even more stable than two separated chemisorbed H* species (2H*). These new stable intermediates have not been considered so far in the analysis of the mechanism and kinetics of the reaction. The work shows that the possible formation of dihydrogen and dihydride complexes is not limited to SACs but is also valid for SAAs and needs to be considered in the study of hydrogen-based reactions on these systems.



 Please wait while we load your content...
Please wait while we load your content...