Mechanistic insights into the conversion of polyalcohols over Brønsted acid sites†
Abstract
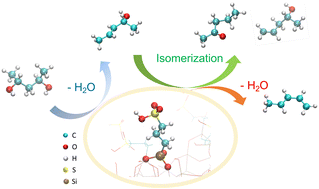
Multi-layered plastic (MLP) films provide excellent properties for food packaging; however, because of the complicated chemistry of the hydrophobic substrates and tightly laminated hydrophilic layers, it is challenging to recycle the MLP films. The selective conversion of polar functional groups in hydrophilic layers with a minimal destruction of the carbon backbone of constituted polymers is considered a promising approach to improve the recyclability of MLP films. In this study, we combine computational and experimental studies to investigate the acid-catalyzed conversion of functional groups in polyalcohols, through which we report potential reaction pathways involved in the conversion of ethylene vinyl alcohol (EVOH), a common copolymer used in MLP films as an oxygen barrier. Using 2,4-pentanediol as a model compound, we show that its conversion proceeds primarily through the dehydration of a hydroxyl group via either a stepwise or a concerted mechanism to form an unsaturated alcohol that can be further converted into a conjugated diene or a saturated ketone; this secondary reaction determines the product selectivity. The rate determining intermediates and transition states are identified, and the activation barriers are calculated, in agreement with experimentally observed product selectivities. This study thus provides insights for the selective conversion of EVOH.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...