Rhodium-catalyzed double hydroboration of pyridine: the origin of the chemo- and regioselectivities†
Abstract
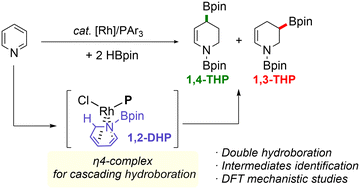
Rhodium-catalyzed hydroboration of unsaturated organic molecules is a well-established reaction and conventionally operates via a Rh(I/III) cycle involving an inner-sphere hydride transfer. Suginome previously applied a Rh–PCy3 catalyst for the hydroboration of pyridines to selectively produce the corresponding 1,2-dihydropyridines. We now found that PPh3 in combination with [Rh(cod)Cl]2 as a precatalyst (P/Rh = 1 or 2, cod = 1,5-cyclooctadiene) brought about a mixture of N-Bpin-1,2,3,4-tetrahydropyridines bearing an sp3 C–B bond in the C4-(G) or C3-(R) position in 60–90% combined yields, representing the first example of double hydroboration of pyridine. A series of catalytic and stoichiometric reactions under variable conditions revealed that: (1) G and R are generated from the 1,2-dihydropyridine intermediate, not the 1,4-dihydropyridine; (2) the ratio of G and R varies from ∼1 : 2 to 5 : 1 depending on phosphine ligands; and (3) the observable Rh species in solution include Rh(PPh3)Cl(H)(Bpin)(Py)n (n = 1 and/or 2; Py = pyridine) as the resting state, in addition to Rh(trans-PPh3)2Cl(H)(Bpin) and Rh(PPh3)(cod)Cl. Density functional theory (DFT) calculations are conducted to propose plausible reaction mechanisms and reveal the origin of the chemo- and the regioselectivity in the consecutive Rh-catalyzed hydroborations to deliver the final products G and R in one-pot.


 Please wait while we load your content...
Please wait while we load your content...