Ruthenium(ii) arene complexes bearing simple dioxime ligands: effective catalysts for the one-pot transfer hydrogenation/N-methylation of nitroarenes with methanol†
Abstract
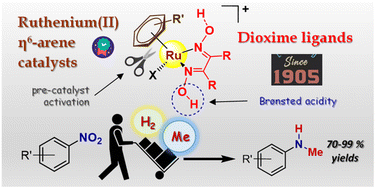
The reductive N-monomethylation of nitroarenes is a reaction of wide scientific interest that can be conveniently realized in one-pot using methanol as the reductant (H2 donor), methylating agent and solvent. Ruthenium(II) η6-arene complexes are increasingly investigated as efficient catalytic precursors for (transfer) hydrogenation and dehydrogenation processes. In this framework, amino or alcohol groups working as proton-relay units are essential to realize a metal–ligand cooperative catalysis. Herein, starting from commercially available and inexpensive dioxime ligands, {RC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(OH)}2, we developed novel ruthenium(II) arene complexes that efficiently catalyze the tandem reduction/N-methylation of aromatic nitrocompounds with methanol. Thus, the complexes [RuX(κ2N-dioxime)(η6-p-cymene)]+ (Cl/dimethylglyoxime, [1]+; Cl/nioxime, [2]+; I/nioxime, [3]+), [RuCl(κ2N-dioxime)(η6-C6Me6)]+ (dimethylglyoxime, [4]+; nioxime, [5]+; diphenylglyoxime, [6]+) and [RuCl(κ2N-nioxime)(η6-1,3,5-C6H3Me3)]+ ([7]+) were isolated as nitrate or hexafluorophosphate salts in high yields and characterized by analytical (CHN content, conductivity) and spectroscopic (IR, NMR) techniques. Furthermore, three zwitterionic oxime–oximato derivatives were prepared (2−H–4−H) and the crystal structures of both dioxime ([3]+, [4]+) and oxime–oximato (4−H) complexes were elucidated by X-ray diffraction. Following optimization of the reaction conditions for the one-pot reduction/N-methylation of nitrobenzene as benchmark substrate (MeOH, tBuOK, 130 °C, 18 h), the catalytic activity of the dioxime complexes [1–7]+ and selected ruthenium(II) compounds for comparative purposes was assessed. The best catalytic precursor, [3]NO3, was effective in the conversion of a series of aromatic nitrocompounds into the respective N-methyl anilines, which were isolated as hydrochloride salts following silica chromatography. NMR and MS experiments carried out on the model catalytic system (nitrobenzene/[3]NO3) confirmed the role of methanol as C1 and H2 source and gave insights about organic intermediates. Following in situ bis-deprotonation to the dioximato complex [3−2H]−, displacement of the η6-arene ligand is proposed as the activation step of the pre-catalyst.
N(OH)}2, we developed novel ruthenium(II) arene complexes that efficiently catalyze the tandem reduction/N-methylation of aromatic nitrocompounds with methanol. Thus, the complexes [RuX(κ2N-dioxime)(η6-p-cymene)]+ (Cl/dimethylglyoxime, [1]+; Cl/nioxime, [2]+; I/nioxime, [3]+), [RuCl(κ2N-dioxime)(η6-C6Me6)]+ (dimethylglyoxime, [4]+; nioxime, [5]+; diphenylglyoxime, [6]+) and [RuCl(κ2N-nioxime)(η6-1,3,5-C6H3Me3)]+ ([7]+) were isolated as nitrate or hexafluorophosphate salts in high yields and characterized by analytical (CHN content, conductivity) and spectroscopic (IR, NMR) techniques. Furthermore, three zwitterionic oxime–oximato derivatives were prepared (2−H–4−H) and the crystal structures of both dioxime ([3]+, [4]+) and oxime–oximato (4−H) complexes were elucidated by X-ray diffraction. Following optimization of the reaction conditions for the one-pot reduction/N-methylation of nitrobenzene as benchmark substrate (MeOH, tBuOK, 130 °C, 18 h), the catalytic activity of the dioxime complexes [1–7]+ and selected ruthenium(II) compounds for comparative purposes was assessed. The best catalytic precursor, [3]NO3, was effective in the conversion of a series of aromatic nitrocompounds into the respective N-methyl anilines, which were isolated as hydrochloride salts following silica chromatography. NMR and MS experiments carried out on the model catalytic system (nitrobenzene/[3]NO3) confirmed the role of methanol as C1 and H2 source and gave insights about organic intermediates. Following in situ bis-deprotonation to the dioximato complex [3−2H]−, displacement of the η6-arene ligand is proposed as the activation step of the pre-catalyst.


 Please wait while we load your content...
Please wait while we load your content...