FDU-12-C encapsulated t-Bu(R,R)CoII(salen) as a cathode catalyst for asymmetric electrocarboxylation of 1-phenylethyl chloride with CO2†
Abstract
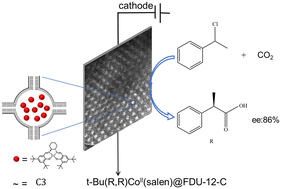
The synthesis of high value-added organic carboxylic acids, especially chiral carboxylic acids, from the electrochemical fixation of CO2 with organic molecules under mild conditions is very important and challenging. In this paper, t-Bu(R,R)CoII(salen)@FDU-12-C composites were prepared as an effective electrocatalytic material for asymmetric electrocarboxylation of 1-phenylethyl chloride. Under optimized conditions, the product, optically active 2-phenylpropionic acid, was obtained with an enantiomeric excess (ee) value of 86% and a yield of 40%. While effectively catalyzing reactions, encapsulated t-Bu(R,R)CoII(salen)@FDU-12-C composites can significantly reduce the amount of chiral t-Bu(R,R)CoII(salen) used, and are easily separated, stable and reusable.


 Please wait while we load your content...
Please wait while we load your content...