Spatial analysis of CO2 hydrogenation to higher hydrocarbons over alkali-metal promoted iron(ii)oxalate-derived catalysts†
Abstract
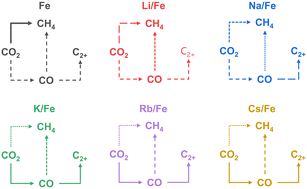
A simple incipient wetness impregnation of ferrous oxalate dihydrate with an aqueous solution of an alkali metal carbonate without any calcination stage results in active and selective catalysts for CO2 hydrogenation to higher hydrocarbons. No catalyst reduction before the reaction is required, because the catalytically active iron carbide and Fe3O4 phases are in situ formed under reaction conditions. The effects of the kind of alkali metal and the amount of Rb on the reaction-induced catalyst restructuring in a spatially resolved manner were thoroughly investigated. The consequences of such changes for catalyst performance were also elucidated. The kind of alkali metal and the amount of Rb were established to mainly hinder the rate of direct CO2 conversion to CH4, while CO hydrogenation to this product is not significantly affected. The dominance of the latter pathway increases along the catalyst bed. The positive promoter effect on the selectivity to higher hydrocarbons is related to the acceleration of the rate of CO hydrogenation to these products.


 Please wait while we load your content...
Please wait while we load your content...