Chemodivergent coupling of azoarenes with benzyl alcohols via a borrowing hydrogen strategy using a well-defined nickel catalyst†
Abstract
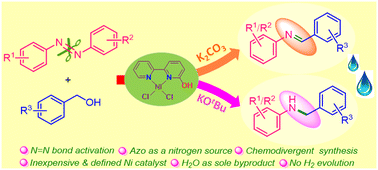
Chemodivergent (de)hydrogenative coupling of azoarenes with benzyl alcohols is achieved via the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond activation using an inexpensive and well-defined (6-OH-bpy)NiCl2 catalyst. This protocol highlights the construction of C–N bonds via a borrowing hydrogen strategy that offers substituted imines and amines. A range of azo compounds couple with various substituted benzyl alcohols in a tandem hydrogenation/dehydrogenation process. The nickel catalyst along with the K2CO3 or KOtBu base governed the selectivity in imine and amine formation. A preliminary mechanistic study establishes the crucial role of metal–ligand cooperation (MLC) comprising the distinct radical pathways.
N bond activation using an inexpensive and well-defined (6-OH-bpy)NiCl2 catalyst. This protocol highlights the construction of C–N bonds via a borrowing hydrogen strategy that offers substituted imines and amines. A range of azo compounds couple with various substituted benzyl alcohols in a tandem hydrogenation/dehydrogenation process. The nickel catalyst along with the K2CO3 or KOtBu base governed the selectivity in imine and amine formation. A preliminary mechanistic study establishes the crucial role of metal–ligand cooperation (MLC) comprising the distinct radical pathways.


 Please wait while we load your content...
Please wait while we load your content...