Abstract
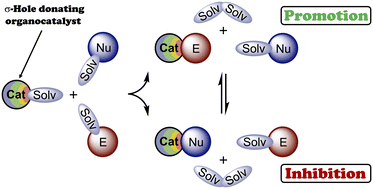
The combination of experimental data and results of DFT calculations indicates that the catalytic activity of chalconium and halonium salts serving as σ-hole donating organocatalysts cannot be clearly estimated via analysis of the electrostatic potential on the catalysts' σ-holes and values of the catalyst⋯TS intermolecular interactions, such as polarization effects, charge transfer, or covalency of bonding. Moreover, the real catalytic effect might not correlate well with the values of Gibbs free energy of activation of the reactions, because solvation effects and other competitive binding processes play at least an equal or even more important role in the catalysis. It was shown in the present work that the solvation can either lead to the increase of equilibrium concentration of reactive catalyst⋯electrophile associates, thus accelerating the reaction, or brings favorable generation of catalyst⋯nucleophile species resulting in the suppression of the catalytic activity of the organocatalyst.


 Please wait while we load your content...
Please wait while we load your content...