Enantioconvergent transformations of secondary alcohols through borrowing hydrogen catalysis
Abstract
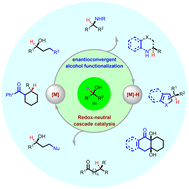
Direct substitution of readily available alcohols is recognized as a key research area in green chemical synthesis. Starting from simple racemic secondary alcohols, the achievement of catalytic enantioconvergent transformations of the substrates will be highly desirable for efficient access to valuable enantiopure compounds. To accomplish such attractive yet challenging transformations, the strategy of the enantioconvergent borrowing hydrogen methodology has proven to be uniquely effective and versatile. This review aims to provide an overview of the impressive progress made on this topic of research that has only thrived in the past decade. In particular, the conversion of racemic secondary alcohols to enantioenriched chiral amines, N-heterocycles, higher-order alcohols and ketones will be discussed in detail.
- This article is part of the themed collection: Metals in Asymmetric Catalysis


 Please wait while we load your content...
Please wait while we load your content...