N-Heterocyclic carbenes as privileged ligands for nickel-catalysed alkene functionalisation
Abstract
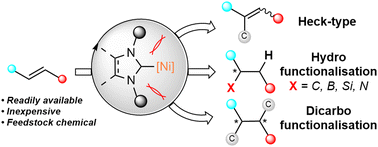
Alkene functionalisation is a powerful strategy that has enabled access to a wide array of compounds including valuable pharmaceuticals and agrochemicals. The reactivity of the alkene π-bond has allowed incorporation of a diverse range of atoms and functional groups through a wide variety of reaction pathways. N-Heterocyclic carbenes (NHCs) are a class of persistent carbenes that are widely employed as ancillary ligands due to their ability to act as strong σ-donors compared to widely-applied conventional phosphine-based ligands. NHCs are also unique as their molecular bulk provides steric influence for regio- and stereo-control in many alkene functionalisation reactions, illustrated by the examples covered in this review. A combination of the unique reactivity of NHC ligands and nickel's characteristics has facilitated the design of reaction pathways that show distinct selectivity and reactivity, including the activation of bonds previously considered “inert”, such as C–H bonds, the C–O bond of ethers and esters, and the C–N bonds of amides. This review summarises the advancements in Ni(NHC) catalysed alkene functionalisation up to 2022, covering the following major reaction classes: Heck-type reactions, hydrofunctionalisation and dicarbofunctionalisation.


 Please wait while we load your content...
Please wait while we load your content...