Effect of cyano-addition on the photoacidity switch in 5-cyano-8-amino-2-naphthol†
Abstract
Cyano- or CN-additions are often utilized in the design of photoacids to enhance and/or enable excited state proton transfer (ESPT) from the protic site to aqueous and nonaqueous solvents. In diprotic photoacid 8-amino-2-naphthol (8N2OH), the protonation state of the amino group (NH3+/NH2) acts as an on–off switch for ESPT at the OH site in water. This study investigated whether the addition of CN in 5-cyano-8-amino-2-naphthol (5CN8) could override this switch and promote new ESPT pathways. Analysis of the steady-state and time-resolved emission data showed that in the presence of protonated NH3+, CN enhances OH photoacidity (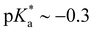 vs.
vs.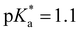 in 8N2OH) and activates the ESPT pathway at NH3+
in 8N2OH) and activates the ESPT pathway at NH3+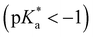 . Both protic sites, OH and NH3+, can also donate a proton to methanol upon excitation. In contrast, in the presence of deprotonated NH2, despite the addition of CN, ESPT is still not observed at the OH site for 5CN8. Thus, the addition of CN cannot override or negate the inhibiting effect of NH2 on OH photoacidity. Potential causes for this inhibition are discussed, including electronic and antiaromaticity effects of CN and NH2 substitution.
. Both protic sites, OH and NH3+, can also donate a proton to methanol upon excitation. In contrast, in the presence of deprotonated NH2, despite the addition of CN, ESPT is still not observed at the OH site for 5CN8. Thus, the addition of CN cannot override or negate the inhibiting effect of NH2 on OH photoacidity. Potential causes for this inhibition are discussed, including electronic and antiaromaticity effects of CN and NH2 substitution.



 Please wait while we load your content...
Please wait while we load your content...