Evidence of extremely short hydrogen bond in the homoconjugated ferrocene-1,1′-diyl-bisphosphinic acid anion: sign change of the H/D isotope effect on the 31P NMR chemical shift†
Abstract
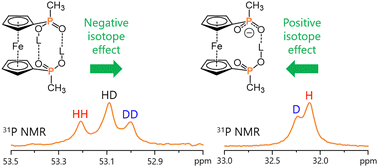
The structure of the two intramolecular hydrogen-bonded motifs within ferrocene-1,1′-diyl-bisphosphinic acid, a cyclic dimer and a homoconjugated anion, has been experimentally revealed by NMR spectroscopy for a solution in a low-freezing polar aprotic CDF3/CDF2Cl medium at 100 K. Structure elucidation was achieved with the help of the H/D isotope effects on the 1H, 2H and, for the first time, 31P NMR chemical shifts. The questions of bridging hydron localization and origins of opposite signs of H/D isotope effects on the 31P NMR chemical shifts in the cyclic dimer and homoconjugated anion have also been addressed by DFT calculations, including numerical solution of the Schrödinger equation for proton and deuteron vibrations in the anharmonic double well potentials.


 Please wait while we load your content...
Please wait while we load your content...