Spin–orbit coupling in low-lying electronic states of CuH†
Abstract
Potential energy curves for the low-lying electronic states of CuH have been calculated using the MRCI method with a large active space containing the 3d, 4s, 4pz and 3d′ orbitals of the copper atom. All electronic states of CuH correlating with the 3d10 4s1 (2S) and 3d9 4s2 (2D) states of Cu were included, and spin–orbit corrections were calculated using the MRCI wavefunctions. Potential energy curves were obtained for individual Ω states, and the Einstein A coefficients were computed for the spin-forbidden transitions from the low-lying electronic states to the X1Σ+ ground state. Diagonal and off-diagonal elements of the spin–orbit Hamiltonian matrix were calculated for the X1Σ+, A1Σ+, 13Σ+, 23Σ+, 11Π, 13Π, 11Δ and 13Δ states, and the mixing of vibronic wavefunctions was investigated using the eigenvectors. The available experimental data on vibrational levels of the A1Σ+ excited state of CuH and CuD were combined in a multi-isotopologue Birge–Sponer plot, and the ab initio data on the unobserved vibrational levels were used for a more accurate extrapolation, resulting in a new experimental value for the equilibrium dissociation energy (De) of the CuH molecule. The De value for the ground state of CuH was determined to be 22 630 ± 250 cm−1, resulting in D0 values of 2.686 and 2.720 eV for CuH and CuD, respectively. Therefore, new experimental values for the bond dissociation enthalpies 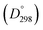 of CuH and CuD are reported to be 262.9 ± 3 and 266.2 ± 3 kJ mol−1, respectively.
of CuH and CuD are reported to be 262.9 ± 3 and 266.2 ± 3 kJ mol−1, respectively.



 Please wait while we load your content...
Please wait while we load your content...