Intramolecular dimer formation reveals anomalous solvation within fluorous solvent perfluorodecalin†
Abstract
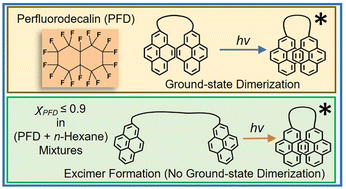
Due to the unusual properties of fluorine, a fluorous solvent obtained by replacing hydrogen atoms with fluorine atoms in a hydrocarbon solvent may afford unusual solute solvation and aggregation. A fluorescent dipyrenyl probe, 1,3-bis(1-pyrenyl)propane (BPP), was utilized to explore intramolecular aggregation within perfluorodecalin (PFD), a prototypical fluorous solvent. Steady-state fluorescence emission and excitation along with excited-state emission intensity decay of BPP were acquired in the temperature range of 293.15 to 333.15 K in fluorous solvent PFD and n-hexane-added PFD. The probe BPP is known to form an intramolecular excimer in common hydrocarbon solvents, which is characterized by a broad structureless low-energy emission band; the formation of intramolecular excimers exclusively in the excited state was confirmed via excitation scans along with the intensity decay of the monomer and excimer, respectively. While intramolecular dimerization of BPP does occur in PFD, emission wavelength-dependent excitation spectra and fits of the intensity decay data reveal that intramolecular aggregation takes place in the ground state as well. This ground-state heterogeneity is reduced as the temperature is increased. In the presence of as small as 0.1 mole fraction of n-hexane, the ground-state aggregation vanishes, and intramolecular dimerization again takes place exclusively in the excited state. The data hint at the poor solvation of BPP in PFD to be responsible for intramolecular ground-state aggregation. In PFD-rich (PFD + n-hexane) mixtures, probe pyrene (Py) was found to be preferentially solvated by n-hexane, further corroborating the above-mentioned proposition. Clear differences in solute solvation within a fluorous solvent as compared to hydrocarbon solvents leading to unexpected solubilization and aggregation in fluorous media are amply demonstrated.


 Please wait while we load your content...
Please wait while we load your content...