Spectroscopic identification of the ammonia–mercapto radical complex†
Abstract
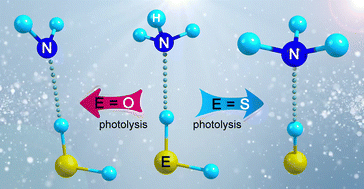
The elusive hydrogen-bonded radical complex (˙SH⋯NH3) consisting of ammonia (NH3) and a mercapto radical (˙SH) has been generated through the 193 nm laser photolysis of the molecular complex between NH3 and hydrogen sulfide (H2S) in solid Ar- and N2-matrixes at 10 K. The identification of ˙SH⋯NH3 with matrix-isolation IR spectroscopy and UV-vis spectroscopy is supported by 15N- and D-isotope labeling experiments and quantum chemical calculations at the B3LYP-D3(BJ)/6-311++G(3df,3pd) level of theory. In line with a large red shift of −172.2 cm−1 for the frequency of the S–H stretching mode observed in ˙SH⋯NH3 (cf. free ˙SH), the radical ˙SH acts as a hydrogen donor, and NH3 acts as an acceptor. According to the calculations at the CCSD(T)/aug-cc-pVTZ level, the SH⋯N bonded structure ˙SH⋯NH3 (binding energy De = 3.9 kcal mol−1) is more stable than the isomeric amidogen radical complex HSH⋯˙NH2 (De = 2.8 kcal mol−1) by 16.6 kcal mol−1. This is in sharp contrast to the photochemistry of the closely related HOH⋯NH3 complex, since the water–amidogen radical complex HOH⋯˙NH2 (De = 5.1 kcal mol−1) was generated under similar photolysis conditions, whereas the ammonia–hydroxyl radical complex ˙OH⋯NH3 (De = 7.9 kcal mol−1) is higher in energy by 9.3 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...