pH dependence of the assembly mechanism and properties of poly(l-lysine) and poly(l-glutamic acid) complexes†‡
Abstract
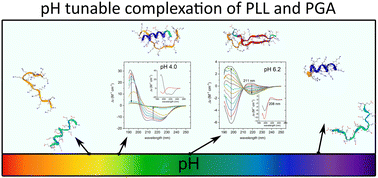
We show by extensive experimental characterization combined with molecular simulations that pH has a major impact on the assembly mechanism and properties of poly(L-lysine) (PLL) and poly(L-glutamic acid) (PGA) complexes. A combination of dynamic light scattering (DLS) and laser Doppler velocimetry (LDV) is used to assess the complexation, charge state, and other physical characteristics of the complexes, isothermal titration calorimetry (ITC) is used to examine the complexation thermodynamics, and circular dichroism (CD) is used to extract the polypeptides’ secondary structure. For enhanced analysis and interpretation of the data, analytical ultracentrifugation (AUC) is used to define the precise molecular weights and solution association of the peptides. Molecular dynamics simulations reveal the associated intra- and intermolecular binding changes in terms of intrinsic vs. extrinsic charge compensation, the role of hydrogen bonding, and secondary structure changes, aiding in the interpretation of the experimental data. We combine the data to reveal the pH dependency of PLL/PGA complexation and the associated molecular level mechanisms. This work shows that not only pH provides a means to control complex formation but also that the associated changes in the secondary structure and binding conformation can be systematically used to control materials assembly. This gives access to rational design of peptide materials via pH control.


 Please wait while we load your content...
Please wait while we load your content...