Solid–liquid phase transition inside van der Waals nanobubbles: an atomistic perspective
Abstract
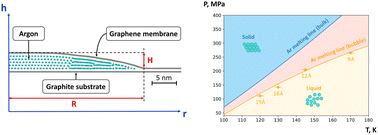
The liquid–solid phase transition during the confinement of a van der Waals bubble is studied using molecular dynamics simulations. In particular, argon is considered inside a graphene bubble, where the outer membrane is a sheet of graphene, and the substrate is atomically flat graphite. A methodology to avoid metastable states of argon is developed and implemented to derive a melting curve of trapped argon. It is found that in the confinement, the melting curve of argon shifts toward higher temperatures, and the temperature shift is about 10–30 K. The ratio of the height to the radius of the GNB (H/R) decreases with increasing temperature. It also most likely undergoes an abrupt change through the liquid–crystal phase transition. The semi-liquid state of argon was detected in the transition region. At this state, the argon structure stays layered, but the atoms travel distances of several lattice constants.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...