Fluorescent nano-sized aggregates of halogen bonded complexes formed using perfluoropropyl iodides: a systematic comparison between two isomeric halogen bond acceptors, aniline and 4-methyl pyridine†
Abstract
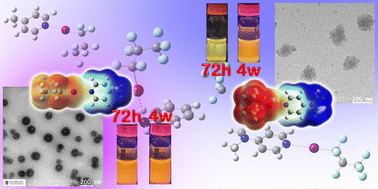
The halogen bonds (XB) formed by the two isomers 4-methyl pyridine (MePy) and aniline (ANL) with heptafluoro-1-propyl iodide (n-C3F7I) and heptafluoro-2-propyl iodide (iso-C3F7I) were investigated using vibrational (FT-IR and Raman) spectroscopy and quantum mechanical calculations. While these two isomers indicated a distinctive impact on the ring related vibrations, molecular electrostatic potential, frontier molecular orbitals, intermolecular electron density delocalisation and consequential charge transfer upon halogen bonding with n-C3F7I and iso-C3F7I, the dramatic intermolecular charge transfer (CT) occurring on the MePy involved XB systems demonstrated an ion-pair like aggregation. Such aggregation, after 72 h and longer after mixing, leads to an emission of fluorescence for both [MePy·C3F7I] systems. The resulting nano-sized aggregates were characterised using UV-Vis absorption and fluorescence spectroscopy along with scanning and transmittance electron microscopy (SEM and TEM), wherein, the XB complex with iso-C3F7I showed a faster and more severe aggregation due to a stronger CT than that with n-C3F7I. The present work is the first case of aggregation induced emission (AIE) due to aggregation of XB complexes formed by small neutral molecules.


 Please wait while we load your content...
Please wait while we load your content...