Alkylphosphonium carboxylate ionic liquids with tuned microscopic structures and properties†
Abstract
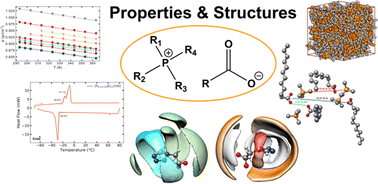
Eleven pure alkylphosphonium carboxylate ionic liquids (ILs) were synthesised following a reliable and accessible route. Tetrabutylphosphonium and tetradecyltrihexylphosphonium cations were associated to a variety of [R–COO]− anions with R varying from shorter to longer linear alkyl chains; smaller to bulkier branched alkyl chains; cyclic saturated aliphatic and aromatic moieties; and one heterocyclic aromatic ring containing nitrogen. A combined experimental and molecular simulation study allowed the full characterization of the physico-chemical properties, the structure and the thermal stability of the synthesized ILs. Although slightly more viscous than their imidazolium counterparts, the viscosities of the prepared salts decrease dramatically with temperature and are comparable to other ILs above 50 °C, a manageable temperature as they are thermally stable up to temperatures above 250 °C, even under an oxidizing atmosphere. The microscopic structure of the phophonium ILs is rich and has been studied both experimentally using SAXS and by molecular dynamics simulation using state of the art polarizable force fields whose parameters were determined when necessary. Unique and surprising anion–anion correlations were found for the tetrazolate-based IL allowing to explain some of the unique physical–chemical properties of this phosphonium salt.


 Please wait while we load your content...
Please wait while we load your content...