A simple topology-based model for predicting the activation barriers of reactive processes at 0 K†
Abstract
This work reveals an underlying correlation between the topology and energetic features of matter configurations/rearrangements by exploiting two topological concepts, namely, structural stability and persistency, leading thus to a model capable of predicting activation energies at 0 K. This finding provides some answers to the difficulties of applying Thom's functions for extracting energetic information of rate processes, which has been a limitation for exact, biological, and technological sciences. A linear relationship between the experimental barriers of 17 chemical reactions and both concepts was found by studying these systems’ topography along the intrinsic reaction coordinate. Such a procedure led to the model 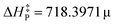 , which accurately predicts the activation energy in reacting systems involving organic and organometallic compounds under different conditions, e.g., the gas-phase, solvent media, and temperature. This function was further recalibrated to enhance its predicting capabilities, generating the equation
, which accurately predicts the activation energy in reacting systems involving organic and organometallic compounds under different conditions, e.g., the gas-phase, solvent media, and temperature. This function was further recalibrated to enhance its predicting capabilities, generating the equation 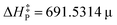 for this procedure, characterized by a squared Pearson correlation coefficient (r2 = 0.9774) 1.1 times higher. Surprisingly, no improvement was observed.
for this procedure, characterized by a squared Pearson correlation coefficient (r2 = 0.9774) 1.1 times higher. Surprisingly, no improvement was observed.



 Please wait while we load your content...
Please wait while we load your content...