Low-temperature kinetics for the N + NO reaction: experiment guides the way†
Abstract
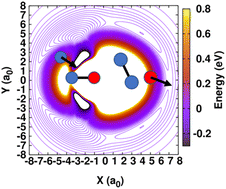
The reaction N(4S) + NO(X2Π) → O(3P) + N2(X1Σ+g) plays a pivotal role in the conversion of atomic to molecular nitrogen in dense interstellar clouds and in the atmosphere. Here we report a joint experimental and computational investigation of the N + NO reaction with the aim of providing improved constraints on its low temperature reactivity. Thermal rates were measured over the 50 to 296 K range in a continuous supersonic flow reactor coupled with pulsed laser photolysis and laser induced fluorescence for the production and detection of N(4S) atoms, respectively. With decreasing temperature, the experimentally measured reaction rate was found to monotonously increase up to a value of (6.6 ± 1.3) × 10−11 cm3 s−1 at 50 K. To confirm this finding, quasi-classical trajectory simulations were carried out on a previously validated, full-dimensional potential energy surface (PES). However, around 50 K the computed rates decreased which required re-evaluation of the reactive PES in the long-range part due to a small spurious barrier with a height of ∼40 K in the entrance channel. By exploring different correction schemes the measured thermal rates can be adequately reproduced, displaying a clear negative temperature dependence over the entire temperature range. The possible astrochemical implications of an increased reaction rate at low temperature are also discussed.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...