Abstract
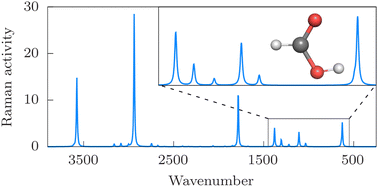
All vibrational energies of the formic acid molecule in different forms (trans-, cis-, delocalized-) were converged up to 4500 cm−1 beyond the zero-point vibrational energy with the GENIUSH-Smolyak variational approach and using an ab initio potential energy surface [D. P. Tew and W. Mizukami, J. Phys. Chem. A, 120, 9815–9828 (2016)]. The full-dimensional dipole and polarizability surfaces were fitted to points computed at the CCSD/aug-cc-pVTZ level of theory. Then, body-fixed vibrational dipole and polarizability transition moments were evaluated and used to simulate jet-cooled infrared and Raman spectra of HCOOH. The benchmark-quality vibrational energy, transition moment, and wave function data will be used in further work for comparison with vibrational experiments, and in further rovibrational computations.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...