Rocking motion in solid proteins studied by the 15N proton-decoupled R1ρ relaxometry†
Abstract
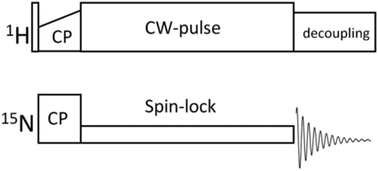
Recently it has been revealed that proteins in solid samples undergo slow overall rocking. The parameters of this motion depend on intermolecular interactions. Therefore, the characterization of the rocking motion enables one to investigate protein–protein interactions. NMR R1ρ relaxometry is the most suitable tool to study slow molecular motions. However, the time scale of the rocking motion is on the edge of the dynamics window of the standard R1ρ experiment, precluding the R1ρ data analysis from being precise and reliable. In this work, we apply a modified R1ρ relaxation method to characterize the slow motion in solids with much higher precision and reliability. The modification is the simultaneous use of a strong 1H-CW pulse and a weak/moderate 15N spin-lock pulse. We demonstrate theoretically and experimentally that under this condition, R1ρ decays have a significantly better signal-to-noise ratio and a much shorter “dead time” caused by the initial oscillations compared to the conventional R1ρ experiment. Moreover, the proton-decoupled R1ρ's can be measured at a much smaller difference between the spin-lock and MAS frequencies; thus, much slower molecular motions can be sampled. The proton decoupling during the 15N spin-lock pulse also suppresses the interfering coherent spin–spin relaxation pathway at low spin-lock fields, which overlaps the Bloch–McConnell (chemical exchange) range of R1ρ dispersions. The proton-decoupled and standard R1ρ experiments were used to study the rocking motion of 15N,2H-enriched protein GB1 in two solid forms, microcrystals and lyophilized amorphous powder. The most striking finding is that the correlation function of this motion consists of two components with very different correlation times, 2–20 μs and a few hundred μs. The rocking motion parameters in microcrystals and powder are quite different, revealing the distinct nature of inter-protein interactions in these two samples.


 Please wait while we load your content...
Please wait while we load your content...